eReg Training & Access
Because eReg is a 21 CFR Part 11 complaint product, all users are required to complete the web-based training from Advarra University and then submit the required documentation to the Rutgers Health Clinical Trials Office.
Please submit all requests with at least 3 business days of lead time.
Monitor Access - Previous eRegulatory User
If you have used Advarra eRegulatory at another site, you may use your existing certificate and do not need to complete the training again.
- Request your eReg account here.
- Select User Access Request > eReg
- For Primary Curriculum, select Advarra eReg 1300: Reviewer (Internal or External Monitor) Curriculum
- For Role & Associated Trainings, select Monitor: Reviewer Curriculum, Navigation, Using Review Sessions
- Attach your Certificate of Completion (login to Advarra University > Completed Courses to obtain your certificate).
- You will receive an email with instructions and your credentials within 3 business days.
Monitor Access - New eRegulatory User
If this is your first time using Advarra eRegulatory:
- Request an Advarra University account here.
- Select User Access Request > Advarra University
- Do NOT request an eRegulatory account at this point.
- After receiving a confirmation email that your Advarra University account has been created, login and navigate to the Store to see a listing of the training sessions.
- Search for Advarra eReg 1300: Reviewer (Internal or External Monitor) Curriculum and add it to your cart. Complete the checkout (for $0).
- This training is expected to take no more than 25 minutes.
- Complete the course and download the certificate of completion.
- Request your eReg account here.
- Select User Access Request > eReg
- For Primary Curriculum, select Advarra eReg 1300: Reviewer (Internal or External Monitor) Curriculum
- For Role & Associated Trainings, select Monitor: Reviewer Curriculum, Navigation, Using Review Sessions
- Attach your Certificate of Completion.
- You will receive an email with instructions and your credentials within 3 business days.
Rutgers / RWJBH / UH / Affiliate Access - With Active Advarra University Account
If you have an Advarra University Account:
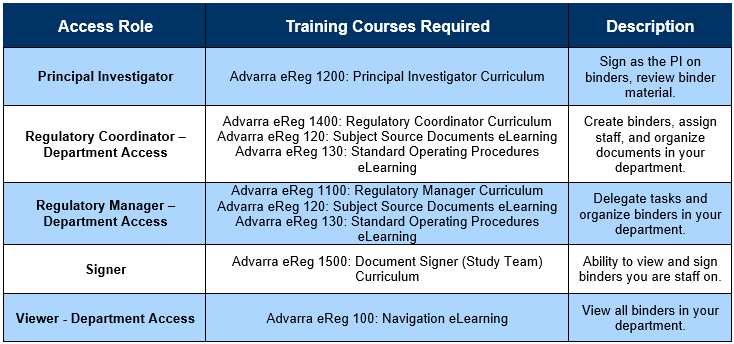
- Identify which eRegulatory Role you should have:
- Login to Advarra University
- Search for the required training courses (as identified in the table above) and add them to your cart. Complete the checkout (for $0).
- Complete the course(s) and download the certificate(s) of completion.
- Request your eReg account here.
- Select User Access Request > eReg
- For Primary Curriculum, add all the curriculums that you completed. Note that you must have completed ALL the required curriculums for your role as identified above.
- For Role & Associated Trainings, select all the courses that you completed.
- Attach your Certificate(s) of Completion.
- You will receive an email with instructions and your credentials within 3 business days.
Rutgers / RWJBH / UH / Affiliate Access - No Advarra University Account
If you do not have an Advarra University Account:
- Request an Advarra University account here.
- Select User Access Request > Advarra University
- Do NOT request an eRegulatory account at this point.
- After receiving a confirmation email that your Advarra University account has been created, login and navigate to the Store to see a listing of the training sessions.
- Identify which eRegulatory Role you should have:
- Search for the required training courses (as identified in the table above) and add them to your cart. Complete the checkout (for $0).
- Complete the course(s) and download the certificate(s) of completion.
- Request your eReg account here.
- Select User Access Request > eReg
- For Primary Curriculum, add all the curriculums that you completed. Note that you must have completed ALL the required curriculums for your role as identified above.
- For Role & Associated Trainings, select all the courses that you completed.
- Attach your Certificate(s) of Completion.
- You will receive an email with instructions and your credentials within 3 business days.
Questions?
Still have questions? Contact the Clinical Trials Office at clinicaltrials@rbhs.rutgers.edu.